Experience Breakthroughs at the Molecular Level
At The Pombo Garcia Lab, we harness the power of STED microscopy to explore the intricacies of cellular structures and biological processes like never before. With our cutting-edge technology, we can visualize cellular components at unprecedented resolution, providing insights that drive innovation in research.
From studying protein interactions to observing live cell dynamics, our STED microscopy capabilities open new avenues in the field of molecular biology. Join us in uncovering the details that shape life.
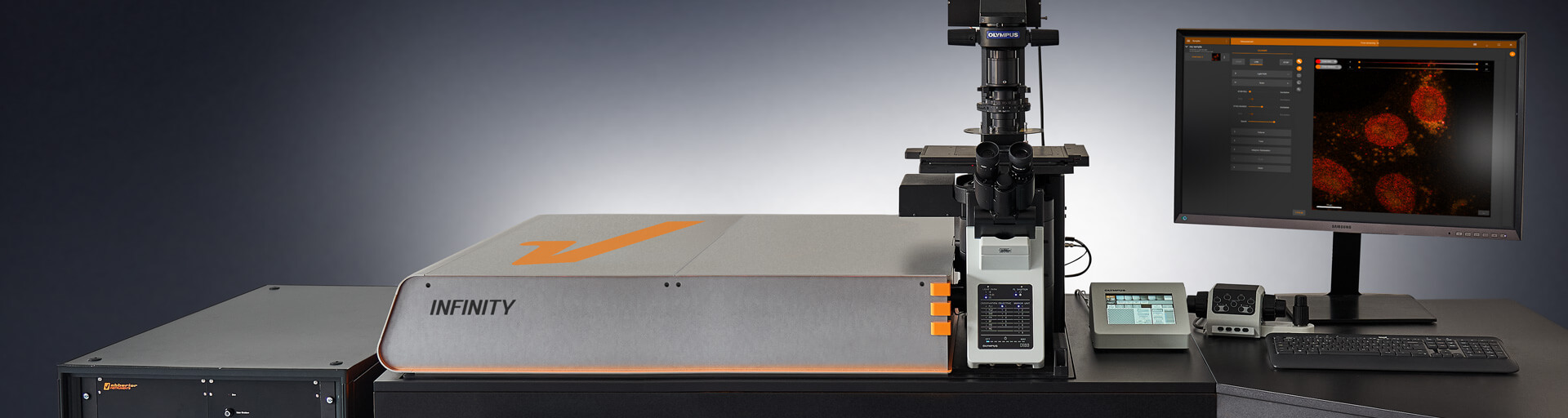
Our In-House Abberior Infinity STED Microscope
STED Microscopy Abberior is most known for STED super-resolution systems. STED uses a depletion laser to selectively turn off fluorescence around the excitation spot, shrinking the effective point spread function. This results in sharp, high-contrast images of structures such as cytoskeletal filaments, nuclear pores, and synaptic proteins.INFINITY Platform Their flagship system, the Abberior INFINITY, is a turnkey, high-end microscope optimized for 2D and 3D STED, confocal, and lifetime imaging. It features:Multiple excitation and depletion lasers.Time-gated detection,Fluorescence Lifetime Imaging (FLIM) ,Spectral separation and multiplexing. Automated alignment and sample feedback control cellular targets, including microtubules, actin, DNA, mitochondria, and lysosomes. The subsequent panels illustrate the application in live or fixed cells. The middle cell diagram highlights the localization of HaloTag-labeled probes (orange circles) to structures resembling microtubules. In the following cell image, the incorporation of multiple fluorophores (e.g., yellow, magenta, and cyan stars) denotes multiplexed labeling of subcellular compartments. The final icon of a microscope signifies the transition to high-resolution imaging. This visual workflow reflects a streamlined pipeline for site-specific, multicolor labeling compatible with super-resolution modalities such as STED, enabling nanoscale visualization of intracellular architecture with high specificity and minimal background. We have the instrument in house available for the students and Staff.
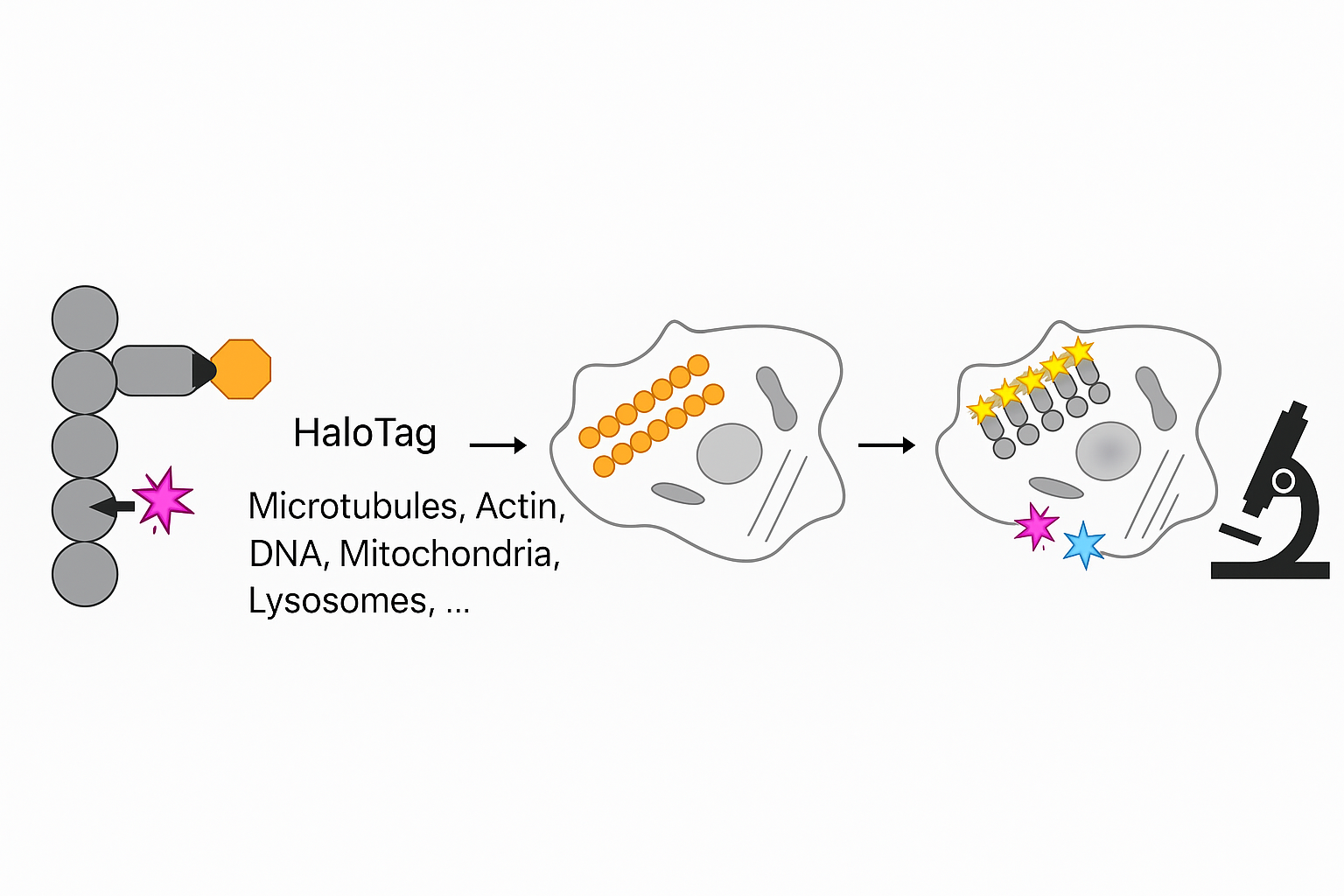
Targeted Protein Labeling for STED Nanoscale Imaging
This schematic illustration demonstrates the use of HaloTag technology for targeted labeling of intracellular components for high-resolution imaging, such as STED (Stimulated Emission Depletion) microscopy. On the far left, a modular protein is shown with a HaloTag domain (depicted as an orange hexagon) and a fluorescent ligand (magenta star) covalently bound to it. This fusion construct can be directed to various cellular targets, including microtubules, actin, DNA, mitochondria, and lysosomes. The subsequent panels illustrate the application in live or fixed cells. The middle cell diagram highlights the localization of HaloTag-labeled probes (orange circles) to structures resembling microtubules. In the following cell image, the incorporation of multiple fluorophores (e.g., yellow, magenta, and cyan stars) denotes multiplexed labeling of subcellular compartments. The final icon of a microscope signifies the transition to high-resolution imaging. This visual workflow reflects a streamlined pipeline for site-specific, multicolor labeling compatible with super-resolution modalities such as STED, enabling nanoscale visualization of intracellular architecture with high specificity and minimal background.
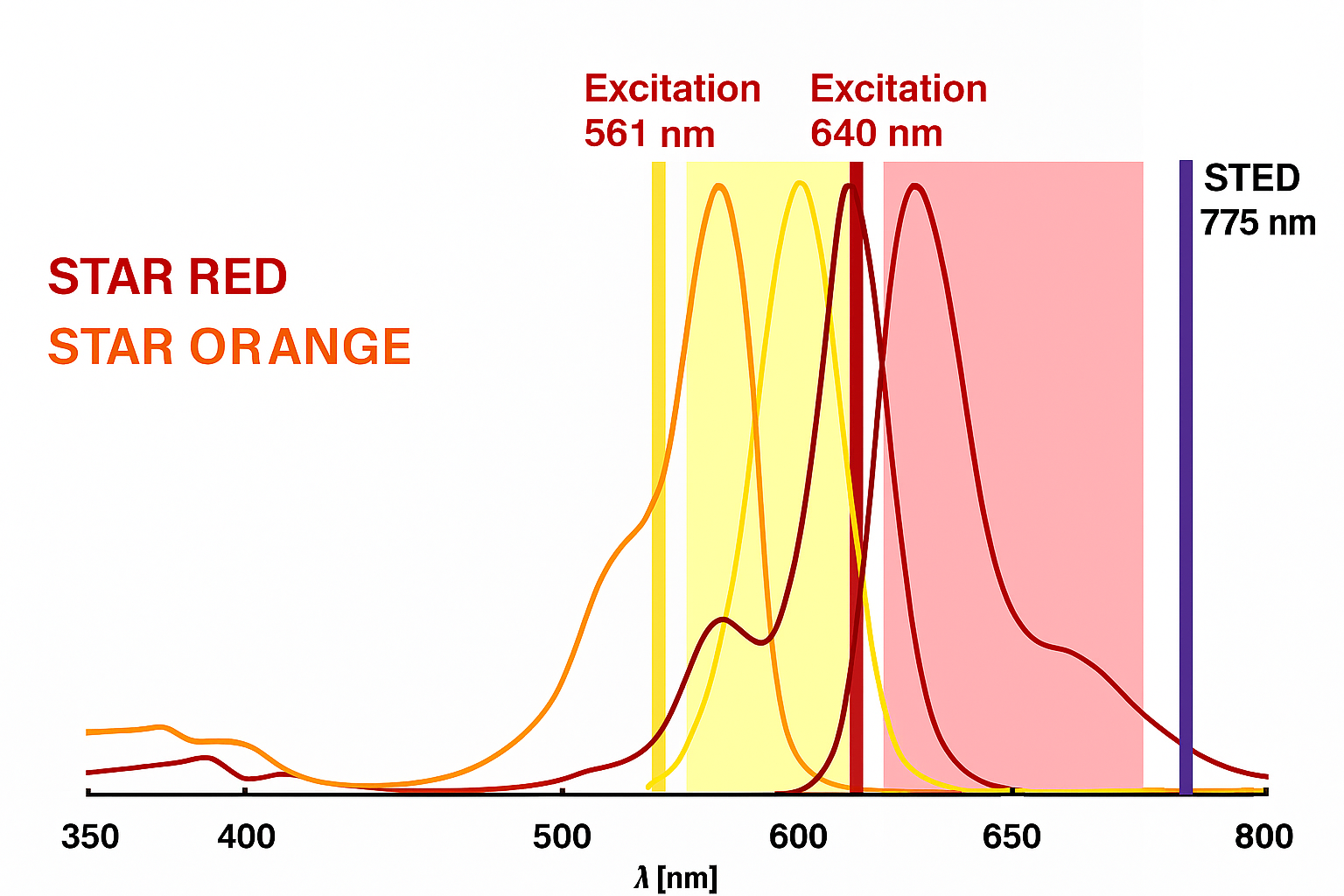
Targeted Protein Labeling for STED Nanoscale Imaging
This schematic illustration demonstrates This graph illustrates the fluorescence excitation and emission profiles of Abberior STAR ORANGE and STAR RED, aligned with STED microscopy parameters. Their complementary spectral properties, minimal overlap, and compatibility with 561 nm, 640 nm, and 775 nm lasers make them ideal candidates for dual-color super-resolution imaging, offering clear spatial separation and high resolution in cellular imaging applications. This spectral setup is ideal for two-color STED nanoscopy, where: STAR ORANGE is used with 561 nm excitation and detected in the 580–620 nm window. STAR RED is used with 640 nm excitation, depleted with 775 nm STED laser, and detected in the far-red range (typically 650–720 nm). The separation in excitation and emission spectra reduces crosstalk, ensuring clean signal separation for multicolor imaging. Both dyes are photostable and optimized for high-intensity STED lasers.
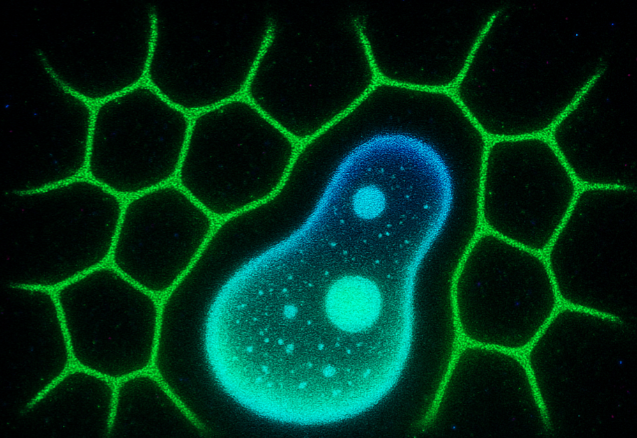
What is LLPS?
Liquid–liquid phase separation (LLPS) is a process by which certain proteins and RNAs spontaneously demix from the surrounding cytoplasm or nucleoplasm to form dense, droplet-like condensates without membranes. Driven by weak, multivalent interactions—often involving intrinsically disordered regions (IDRs) and RNA—LLPS underlies the formation of dynamic structures like nucleoli, stress granules, and P bodies. These condensates are reversible, selective, and responsive to environmental cues. While essential for organizing biochemical reactions, dysregulation of LLPS is linked to diseases such as ALS, cancer, and neurodegeneration. LLPS is commonly studied using fluorescence microscopy, FRAP, and phase diagrams to characterize its dynamic and biophysical properties.