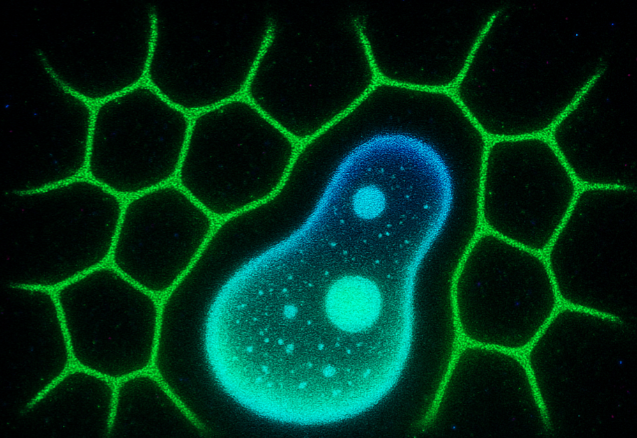
Intra-condensate demixing of TDP-43 inside G3BP1-RNA condensates
Let’s understand condensates
Inside our cells, there’s a lot going on—genes are being read, proteins are being made, and messages are being sent. To keep things organized, cells use special compartments called biomolecular condensates. These are tiny droplets made up of proteins and RNA that group together without needing a surrounding membrane (unlike classic structures like the nucleus or mitochondria). Think of them like oil droplets forming in water—they come together naturally and can dissolve just as easily. This allows cells to quickly adapt to stress, regulate gene activity, or control the flow of biological information. Researchers are discovering that when these droplets don’t work properly—when they become too sticky or don’t dissolve as they should—they may contribute to diseases like cancer, Alzheimer’s, or ALS.
Medium expression of TDP-43ΔNLS undergoing intra-condensate demixing inside stress granules in HeLa cells (
Let’s be more intricate
Biomolecular condensates are dynamic, membraneless organelles formed through liquid–liquid phase separation (LLPS), often driven by weak, multivalent interactions among intrinsically disordered regions (IDRs), low-complexity domains, and RNA-binding motifs. These phase-separated compartments concentrate specific macromolecules to regulate diverse cellular functions such as transcriptional regulation (e.g., super-enhancers), RNA metabolism (e.g., stress granules, nucleoli), and signal transduction (e.g., LAT clusters). Their formation is sensitive to changes in concentration, post-translational modifications, and biophysical parameters such as pH or ionic strength. Emerging evidence links aberrant condensate behavior—such as pathological solidification or altered material states—to neurodegeneration (e.g., FUS and TDP-43 aggregation in ALS), oncogenic signaling (e.g., mutant SHP2 condensates), and viral replication factories. A mechanistic understanding of the molecular grammar underlying condensate assembly and dissolution is crucial for uncovering new therapeutic targets and engineering synthetic cellular organization.
Let’s unlock the potential of Condensates
The potential of biomolecular condensates in future research is vast and transformative. These dynamic, membrane-less structures offer a fundamentally new way of understanding intracellular organization—one that emphasizes physical principles like phase separation rather than classic compartmentalization. By controlling the local concentration and interaction dynamics of key proteins and RNAs, condensates enable cells to rapidly and reversibly coordinate complex processes such as gene expression, signal transduction, and stress response. Their tunable, stimulus-responsive nature makes them not just a fascinating subject of study, but a powerful framework for synthetic biology, where designer condensates could act as programmable cellular circuits or responsive molecular machines. In biomedicine, the implications are profound. Aberrant phase behavior is already implicated in neurodegeneration, cancer, and viral replication, positioning condensates as both biomarkers and therapeutic targets. Drugs could be developed to modulate the material properties of pathological condensates—dissolving them, hardening them, or preventing their formation entirely. Moreover, condensates offer a novel frontier for precision medicine: treatments tailored not only to genetic mutations but to the altered biophysical behavior of intracellular components. Looking forward, harnessing condensates could revolutionize RNA therapeutics, cell reprogramming, organoid engineering, and the creation of adaptive, bio-inspired materials, placing them at the nexus of next-generation biology and engineering.